How to Calculate Neutron Number
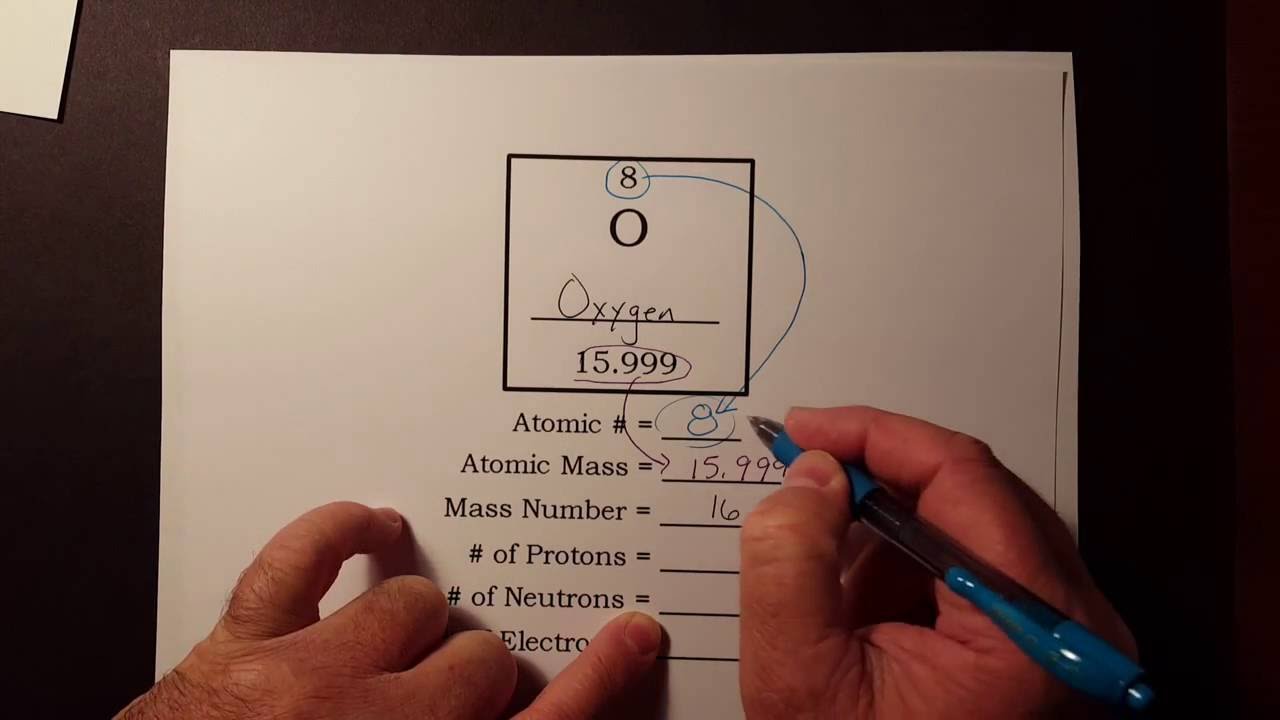
X is the relative abundance. The atomic number of an element is equal to the number of protons in its nucleus.

Counting Atoms Protons Neutrons Electrons Worksheet Counting Atoms Text Features Worksheet Worksheets
M2 is the mass of the second isotope.
. The average isotopic mass. Suppose you have a chlorine 35 and 37. Formula to calculate relative abundance.
ME is the atomic mass of the element from the periodic table. Isotopes are variants of a particular chemical element which differ in neutron number and consequently in nucleon number. In a neutral atom the number of protons equals the number of electrons in shells which is the energy level around the nucleus.
Isotopes are atoms with the same atomic number but distinct neutron numbers and hence distinct mass numbers. M1 is the mass of one isotope.

Definition Of Mass Number With Examples Isoptopes Mass Number Definition Of Mass Atomic Mass Unit

Isotope Atoms With The Same Number Of Protons Atomic Number But Different Atomic Masses Chemistry Education Teaching Science Chemistry Labs

How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
No comments for "How to Calculate Neutron Number"
Post a Comment